Is Al A Transition Metal
Ever found yourself staring at the periodic table, wondering about the quirky characters and their classifications? It's like a cosmic yearbook for elements! Today, we're diving into a question that might seem a little niche, but trust us, it's surprisingly fun and can actually shed some light on some really cool science: Is Aluminum a Transition Metal? This isn't just about memorizing facts; it's about understanding how the elements are organized and why that matters. Think of it as unlocking a secret code that explains so much about the world around us, from the shininess of your kitchen foil to the strength of an airplane wing.
Understanding element classifications, like whether Aluminum (Al) fits the bill for a transition metal, is like having a map to the building blocks of the universe. It helps scientists predict how elements will behave, what kinds of compounds they'll form, and what amazing applications they can have. For us everyday folks, it's a peek behind the curtain, revealing why certain materials are used for specific jobs. It’s the kind of knowledge that makes you feel a little bit smarter with every observation, and frankly, it’s pretty darn fascinating!
The Great Element Debate: Aluminum's Identity Crisis
So, let's get straight to the heart of the matter: is Aluminum a transition metal? The short answer, and the one most chemists will give you, is no. But why? What makes an element a "transition metal" anyway? This is where the periodic table's layout becomes our guide.
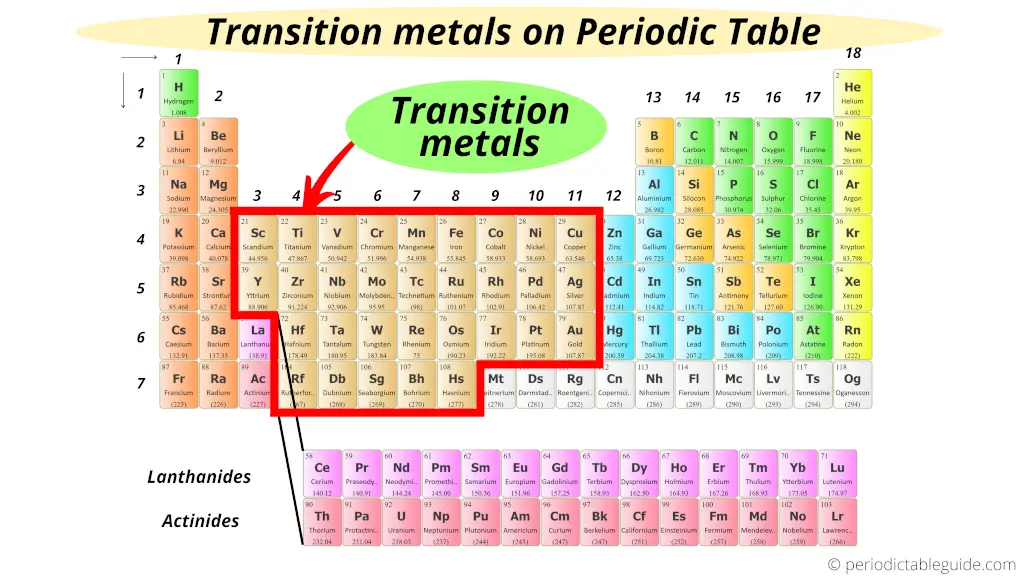
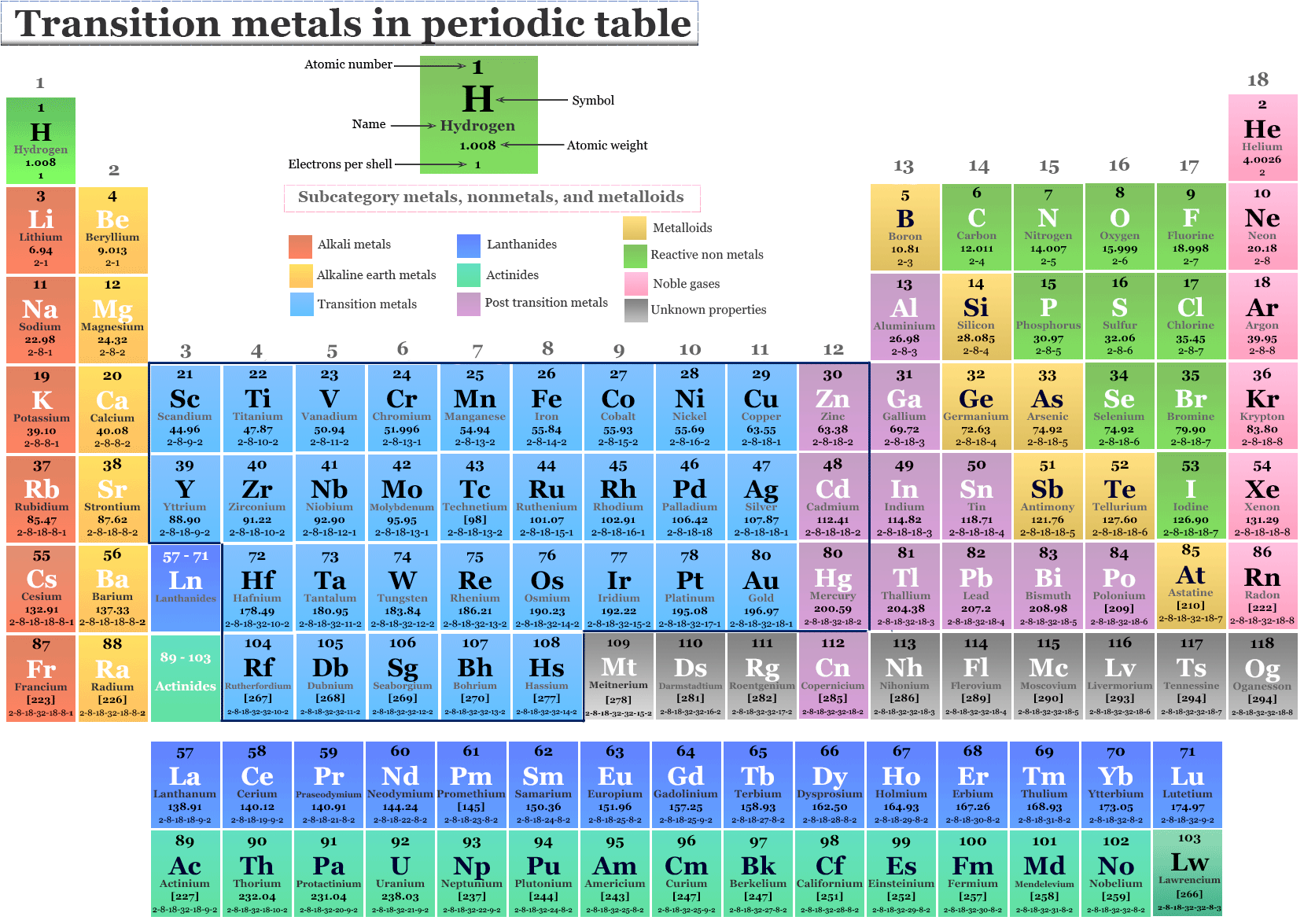
Transition metals are typically found in the d-block of the periodic table, spanning groups 3 through 12. These elements are characterized by having partially filled d orbitals. This electronic configuration is the secret sauce that gives transition metals many of their unique and useful properties. They are often known for forming colorful compounds, exhibiting variable oxidation states (meaning they can lose different numbers of electrons), and acting as excellent catalysts in chemical reactions. Think of familiar friends like iron (Fe), copper (Cu), and gold (Au) – these are classic transition metals, responsible for everything from rust and plumbing to jewelry and electronics.
Now, where does Aluminum (Al) hang out on this grand chart? Aluminum is located in Group 13 and the third period. It's part of the p-block elements. Its electron configuration shows that its outermost electrons are in the p orbitals, not the d orbitals. Specifically, Aluminum has the electron configuration [Ne] 3s² 3p¹. When it forms ions, it readily loses its three valence electrons to become Al³⁺, a stable ion with a full outermost electron shell. This behavior is typical of post-transition metals or other metals, a category that includes elements like gallium (Ga) and indium (In).
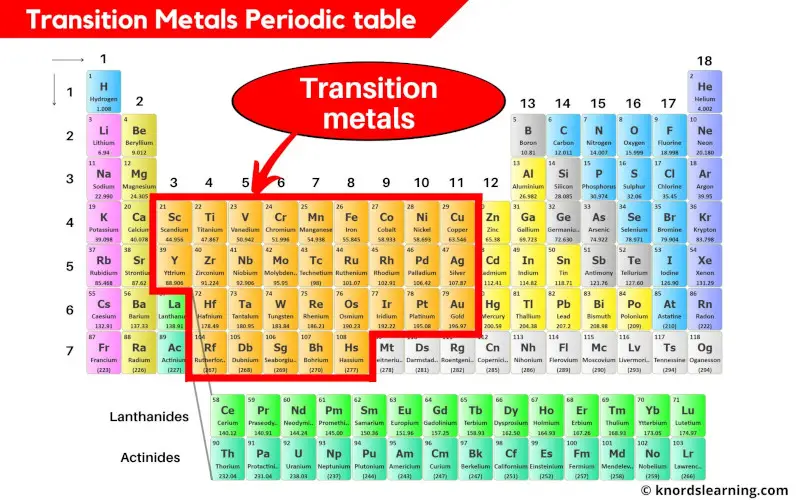
The defining characteristic of transition metals lies in their electron configuration, particularly the involvement of their d-orbitals. Aluminum, lacking these partially filled d-orbitals, doesn't fit the traditional definition.
So, while Aluminum is definitely a metal – it’s shiny, a good conductor of heat and electricity, and malleable – it doesn't possess the electronic structure that defines a transition metal. This might seem like a minor detail, but it explains why Aluminum behaves differently from its transition metal cousins. For instance, Aluminum typically exhibits only one stable oxidation state (+3), unlike many transition metals that can switch between multiple oxidation states.
The Perks of Being (or Not Being) a Transition Metal
Why does this classification matter? Well, understanding these categories helps us predict and utilize the properties of elements. Aluminum's classification as a post-transition metal means it's lightweight, corrosion-resistant (thanks to its protective oxide layer), and abundant. These traits make it incredibly valuable in a vast array of applications.
Consider the aerospace industry, where Aluminum alloys are indispensable for building aircraft due to their strength-to-weight ratio. In construction, its durability and resistance to the elements make it a popular choice for windows, doors, and siding. And, of course, in our kitchens, from foil to cookware, Aluminum's conductivity and ease of shaping are unmatched. These are benefits derived from its specific electron configuration and metallic bonding, even if it doesn't wear the "transition metal" badge.
On the flip side, transition metals, with their variable oxidation states and ability to form complex ions, are crucial for catalysis (think catalytic converters in cars), pigments (the vibrant colors in paints and inks), and biological processes (like the role of iron in our blood or cobalt in vitamin B12).
So, while Aluminum might not be a transition metal by the strictest definition, its metallic properties and unique characteristics make it an equally important and fascinating element on the periodic table. It’s a reminder that the beauty of chemistry lies not just in rigid definitions, but in understanding the nuances that lead to the incredible diversity of materials we see and use every single day. The periodic table, in all its glory, continues to surprise and delight with the stories each element has to tell!