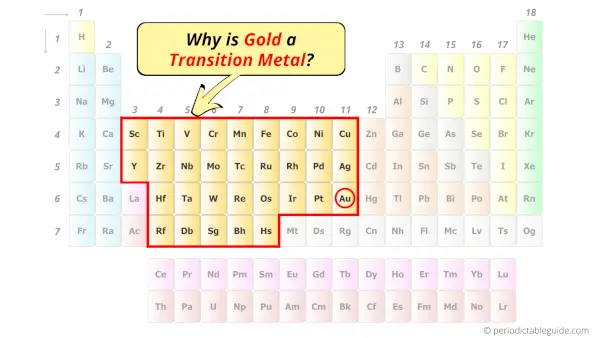
Is Gold A Transition Metal

Hey there, curious minds! Ever wondered about the bling that makes us go "Ooh!" and "Aah!"? We're talking about gold, that sunny, dazzling element that’s been captivating humans for, like, forever. But here’s a sparkly little secret: is this precious metal a diva in its own little club, or does it hang out with the cool kids? Let's dive in and uncover the truth behind gold's place in the grand periodic table party!
Imagine the periodic table as a giant, super-organized party. We've got all sorts of guests: the super-light and bouncy ones, the chill and aloof ones, and then there are the ones who are, well, a little bit of both. They’re the ones who can shift their style, change their vibe, and generally be pretty darn adaptable. Think of them as the chameleons of the elemental world!
Now, where does our beloved gold fit into this shindig? Does it have a VIP pass to a special section, or is it mingling and making friends with everyone? The answer, my friends, is as radiant and delightful as gold itself!
So, Is Gold a Transition Metal? Let's Find Out!
Prepare yourselves, because the answer is a resounding... YES! Our shimmering friend gold is indeed a transition metal. And not just any transition metal, mind you. It’s one of the rockstars of this group!
Think of transition metals as the versatile performers of the periodic table. They’ve got this amazing ability to play multiple roles, almost like actors who can nail a comedy, a drama, and a musical all in one go. They're known for their cool properties, like conducting electricity like a superhero, being shiny enough to blind a dragon, and often forming colorful compounds that look like a painter’s wildest dreams.

Gold, with its undeniable luster and resistance to turning into a rusty mess, totally fits this description. It’s not just sitting pretty; it’s actively participating in the chemical scene with flair! It's like the celebrity guest at the party who's not only gorgeous but also incredibly talented and charming.
What Makes Gold a "Transition" Metal Anyway?
The term "transition" in transition metal refers to a specific quirk in how their electrons behave. These electrons are like tiny dancers in the atom, and in transition metals, some of these dancers are a bit more free-spirited. They can move around and fill up different "seats" in the atomic stadium, which is what gives these metals their unique talents.

Gold's electron arrangement is particularly neat. It has those special outer electron shells that aren't completely full. This "almost full" status is the secret sauce that allows gold to participate in chemical reactions in interesting ways and to exhibit its fabulous properties. It's like having a few empty seats at the concert, so you can easily invite more people to join the band!
This electron dance is what makes gold so stable, which is why it’s been prized for jewelry and coins for millennia. It doesn’t easily rust or corrode, unlike some of its grumpier elemental neighbors. It’s the friend who shows up on time, looks amazing, and never complains about the weather!
Think of it this way: other metals might be like those one-trick ponies, good at one thing and that’s it. Transition metals, however, are like Swiss Army knives of the element world. They’ve got multiple tools and can tackle various chemical situations with grace and power. And gold? It’s definitely the fanciest blade on that knife!
So, when you see that gleaming gold ring or that historical gold coin, you're not just looking at pretty metal. You're looking at a prime example of a transition metal showing off its scientific superpowers! It's chemistry in action, looking absolutely fabulous while doing it.

The fact that gold is a transition metal explains why it's so good at conducting electricity. This is why it’s used in high-end electronics, from your smartphone to super-computers. It’s the silent, shiny hero working behind the scenes to keep our gadgets buzzing. Imagine your phone trying to run without gold connectors – it would be like trying to have a dance party without music!
And the colors! While pure gold is that familiar yellow, when it mixes with other metals, it can create stunning shades of rose and white. This color-changing magic is another hallmark of transition metals, thanks to those adaptable electrons. It’s like gold has a secret wardrobe, ready to switch its hue for any occasion.
So, next time you admire a piece of gold, remember its incredible journey through the periodic table. It’s not just a treasure; it’s a testament to the fascinating science of transition metals, displaying a dazzling array of properties that have made it invaluable throughout history. It’s a true element of elegance and utility, all rolled into one!
Isn’t science amazing? It turns even the most familiar objects, like a piece of gold jewelry, into a whole new world of wonder. Now you can impress your friends with your newfound knowledge about gold's classification. You're basically a mini-scientist, and that's pretty darn cool!
Keep exploring, keep questioning, and always remember that even the most dazzling elements have a fascinating story to tell. And gold, as a proud transition metal, has a story that sparkles brighter than most! It’s a reminder that beauty and brains often go hand-in-hand, especially in the world of chemistry.