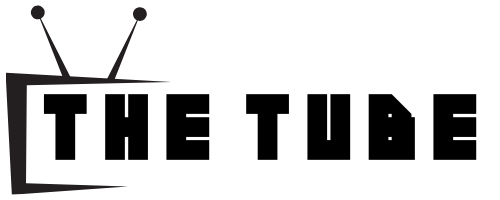Phase Diagram For Carbon Steel

Ever looked at a trusty frying pan and wondered about the secret life of your favorite spatula? Or maybe you've marveled at the sheer strength of a skyscraper's steel frame and thought, "How does it do that?" Well, get ready to have your mind gently tickled by the surprising inner world of carbon steel, specifically its phase diagram. Now, I know what you're thinking: "Phase diagram? Sounds like something a grumpy scientist in a lab coat invented." But stick with me, because this is less about complex equations and more about a little dance of atoms, a subtle shapeshifting that makes your everyday steel objects so darn good at what they do.
Imagine you have a bunch of tiny building blocks – those are our iron atoms. Now, sprinkle in a few smaller, energetic guests – the carbon atoms. These little guys are like the life of the party, able to wiggle their way into the iron's structure and make things a whole lot more interesting. The phase diagram is basically a map that shows us where these atoms like to hang out and what kind of structures they form depending on two main things: how much carbon is present and how hot everything gets.
Think of it like a recipe for steel. If you add just a pinch of carbon (less than about 0.02%), you get something pretty much like pure iron. It's nice and ductile, good for bending and shaping, but not exactly ready to hold up a bridge. This is where we meet our first cozy little spot on the phase diagram, the ferrite phase. Ferrite is like the chill, laid-back friend of the steel world. It's soft, it's forgiving, and it's happy to just exist. Your basic, old-school nails might have a good chunk of ferrite in them.
As you add a bit more carbon, things start to get more exciting. We introduce another character, cementite. Cementite is the complete opposite of ferrite – it's super hard and brittle, like a tiny, angry little knight guarding its territory. When iron and carbon team up in just the right proportions, they can form this formidable compound. But here's where the magic happens: sometimes, ferrite and cementite get together and form a beautifully layered structure called pearlite. Imagine microscopic layers of soft ferrite and hard cementite, like a delightful, metallic sandwich. This is where a lot of the everyday strength of steel comes from. It’s strong but also has a bit of give, making it perfect for things like bicycle frames or even the blades of your favorite knives.

Now, what happens when we crank up the heat? This is where the phase diagram really starts to show off. When you heat steel up, the atoms start to get a bit restless. They jiggle and vibrate with increasing enthusiasm. If you heat it high enough, past a certain point called the A3 line (don't worry about the "A," it's just a marker), the structure starts to change again. The iron atoms decide to rearrange themselves into a new, even more tightly packed, high-temperature form called austenite. Austenite is like the super-friendly, welcoming club of the steel world. It can dissolve a lot more carbon than ferrite can. Think of it as a big, warm hug for the carbon atoms, where they can spread out and be happy.
This transformation is where the real fun begins, like a secret meeting of the steel atoms deciding to throw a party!
The really heartwarming part is what happens when you cool austenite down. How fast you cool it dictates what kind of structure you get. If you cool it slowly, it has time to relax and re-form into ferrite and cementite, eventually giving you pearlite again. But if you cool it really fast – a process called quenching – the carbon atoms don't have time to get out of the way. They get trapped in this super-stressed, distorted structure. This trapped-up state is called martensite. Martensite is like a superhero in disguise. It's incredibly hard and strong, but it's also a bit grumpy and brittle. It's the result of a hurried transformation, a bit like trying to cram for an exam the night before – effective, but not always the most graceful.

And that's where the story gets really sweet. After you've made that super-hard martensite, you can go back and give it a little bit of a reprieve, a gentle warm-up called tempering. This isn't a full-blown transformation, more like a soothing spa treatment for the steel. The tempering process allows some of the trapped carbon atoms to relax a little, making the steel less brittle and more tough, while still keeping a lot of that awesome hardness. It’s like our superhero, after saving the day, getting a nice cup of tea and a biscuit to recover.
So, the next time you admire a perfectly forged sword, a sturdy bridge, or even the simple beauty of a well-made kitchen knife, remember the secret life of its carbon steel. It’s a story of atoms dancing, shifting, and transforming based on heat and composition, all orchestrated by the elegant logic of the phase diagram. It’s a testament to how these humble elements, when guided by understanding and a touch of heat, can create materials that are both incredibly strong and surprisingly nuanced. They’re not just metal; they’re tiny, well-behaved atomic communities working together to serve us in countless ways.