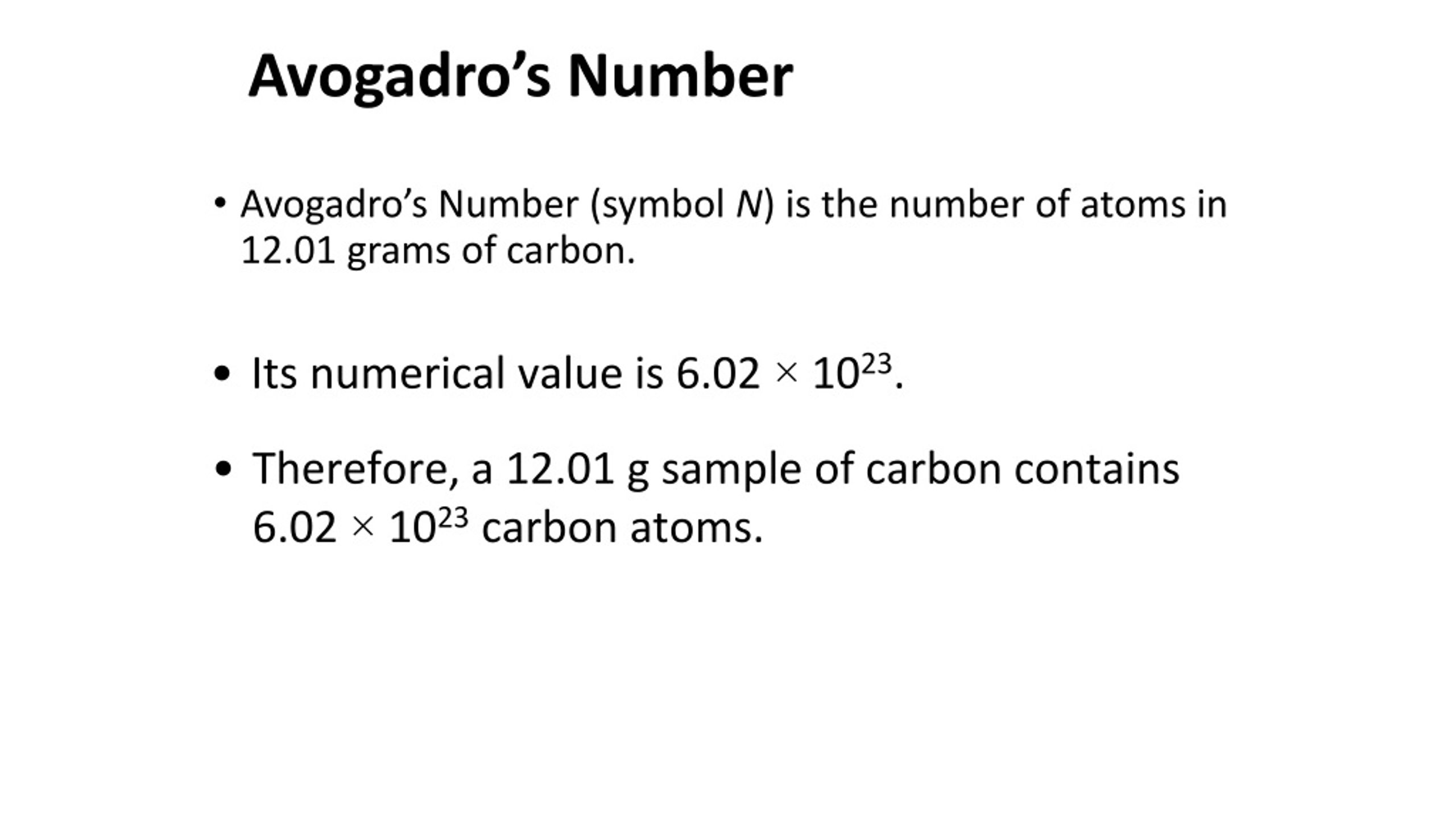
What Is The Numerical Value Of Avogadro's Number
+is+the+number+of+atoms+in+grams+of+carbon.+Its+numerical+value+is+6.02+×.jpg)
Imagine you’re baking cookies, a really, really big batch. We’re not talking about a dozen or two. We’re talking about a batch so enormous it would fill every single house on your street, every single street in your town, and then some. Now, imagine trying to count every single grain of sugar you’d need for that epic cookie-fest. It would take forever, right? Well, scientists face a similar challenge, but instead of sugar, they’re counting teeny-tiny building blocks of everything: atoms and molecules.
And to help them out with this ridiculously large counting job, they have a superhero number. This number has a name that sounds a bit like a fancy Italian dish: Avogadro’s Number. Let’s dive into what this giant number actually is, and why it’s kind of a big deal (literally!).
The Number That’s Bigger Than Your Wildest Dreams
So, what is this magical number? Drumroll, please… It’s approximately 602,214,076,000,000,000,000,000. Phew! Say that ten times fast! For simplicity, scientists often just say 6.022 x 1023. That little “x 1023” is basically a shortcut for adding 23 zeros after the 6.022. It’s like saying “a bazillion gazillion” but with way more precision.
Let’s try to wrap our heads around this number. If you were to count one atom every single second, starting from the Big Bang (that’s about 13.8 billion years ago), you wouldn’t even be close to reaching Avogadro’s Number. You’d still be counting for a very long time. It’s like trying to empty the ocean with a teacup – it’s just that much stuff!
Why Do We Even Need Such a Big Number?
You might be wondering, “Why on earth do we need such a ridiculously large number?” It all comes down to the fact that atoms and molecules are incredibly, unbelievably small. Like, so small you’d need a super-duper microscope to even see one. If you looked at a tiny speck of dust, it contains billions upon billions of these little guys.
.webp)
Scientists work with materials in grams and kilograms, which seem like normal amounts to us. But when you’re dealing with the building blocks of matter, those normal amounts are actually teeming with an unfathomable number of atoms and molecules. Avogadro's Number acts as a bridge between the world of what we can see and weigh, and the invisible world of atoms and molecules. It tells us, for instance, that in a specific amount of a substance (like 12 grams of carbon), there are always this exact same, humongous number of atoms.
A Little Bit of History and a Whole Lot of Fun
The number is named after an Italian scientist called Amedeo Avogadro. He was a bit of a trailblazer back in the 1800s. He figured out some really important stuff about gases, like how they behave and how their particles are arranged. He didn’t discover the exact numerical value of his namesake number himself – that took a lot more clever experimentation over many years by many different scientists. But his ideas were so foundational that the number became known as Avogadro's Number.

Imagine a world before we really understood how much stuff was in a spoonful of water. It would be like trying to cook without measuring cups! Scientists needed a way to count these tiny particles consistently, and Avogadro’s work gave them the perfect starting point. It’s like he gave them the secret handshake to the atomic world.
The Most Popular "Dozen" Ever
Think of it this way: a dozen is 12. A baker’s dozen is 13. These are convenient numbers for counting things we can see and hold. Avogadro’s Number is like the ultimate "dozen" for chemists and physicists. They call a collection of things equal to Avogadro's Number a mole. So, a mole of anything has that enormous number of particles.
A mole of water molecules? That’s 6.022 x 1023 water molecules. A mole of gold atoms? That’s 6.022 x 1023 gold atoms. It’s a unit that helps them talk about incredibly small things in manageable chunks. It’s like saying "a six-pack of soda" instead of "six cans of soda." Except, instead of six, it's a number so big it makes your brain do a little happy dance.
The Heartwarming Connection
What’s truly heartwarming about Avogadro’s Number is that it connects us to the very essence of what we are. Every breath you take, every sip of water, every bite of food – it’s all made up of atoms and molecules. And the quantities of these tiny particles in everyday objects are all governed by this giant number. It’s a silent, constant reminder of the incredible complexity and beauty of the universe, all packed into the smallest of things.
So, the next time you see a science show or hear about atoms, remember Avogadro’s Number. It’s not just a string of digits; it’s a symbol of human curiosity, ingenuity, and our never-ending quest to understand the world around us, one incredibly large number at a time. It’s a testament to how much "stuff" there is, even in the smallest, most invisible corners of our reality. And that, in itself, is pretty amazing, don't you think?