Why Does Fluorine Have A Smaller Atomic Radius Than Oxygen
Hey there, curious minds! Ever wonder why some things in the universe are a little bit… well, smaller than others? Today, we’re going to dive into a tiny mystery that actually has some pretty big implications for our everyday lives. We’re talking about two elements, fluorine and oxygen, and why fluorine, despite being right next to oxygen on the periodic table, is a bit more petite.
Now, I know what you might be thinking: “Periodic table? Atomic radius? Is this going to be a lecture?” Absolutely not! Think of this more like a friendly chat over a cup of coffee, where we unravel a cool science tidbit. We’re not aiming for rocket science here, just a little peek into the amazing world of atoms.
The Atomic Shrink-Ray Mystery
Imagine you’re looking at two houses on the same street. They’re pretty similar, but one seems just a smidge more compact. Why? Maybe the builder used slightly different materials, or perhaps the layout is just a little more efficient. Atoms are a bit like that, but instead of bricks and mortar, we’re dealing with protons, neutrons, and electrons.
Our stars today are fluorine (F) and oxygen (O). You know oxygen, right? It’s that stuff we breathe! And fluorine? Well, you might know it from toothpaste – it’s what helps keep our pearly whites strong.
So, the question is: Why is fluorine smaller than oxygen? It’s like asking why a cozy studio apartment is smaller than a slightly larger one-bedroom, even though they’re both in the same building complex. Let’s break it down.
Meet the Nucleus: The Atom’s Tiny Boss
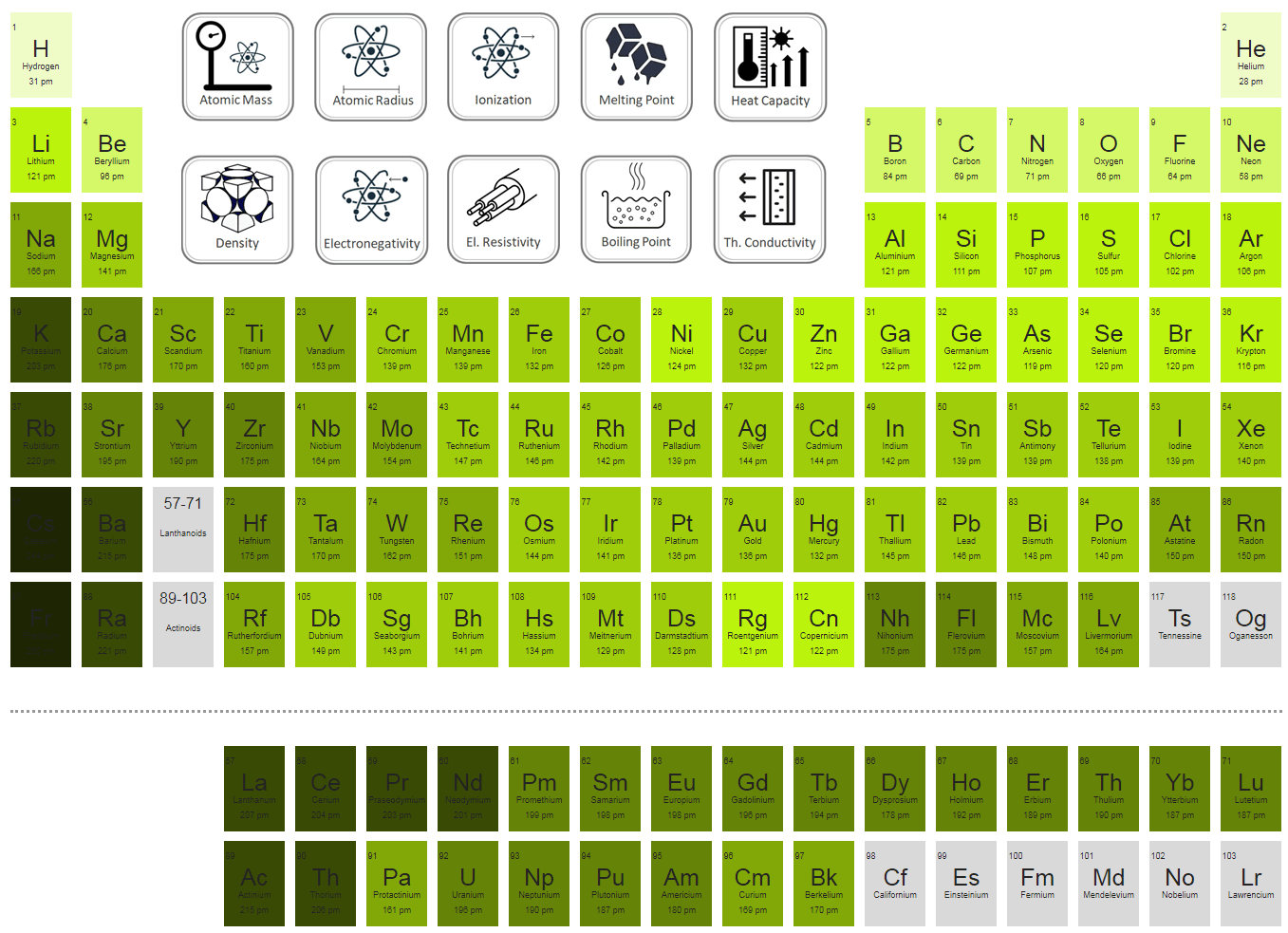
At the very heart of every atom is the nucleus. This is like the control center, packed with positively charged particles called protons. The number of protons is what defines an element. Oxygen has 8 protons, and fluorine has 9 protons. So, fluorine has one more proton than oxygen.
Now, here’s where things get interesting. These protons, with their positive charge, have a really important job: they pull on the negatively charged electrons that whizz around the nucleus. Think of it like a miniature solar system, where the nucleus is the sun and the electrons are planets. The stronger the sun’s gravity (the more protons), the tighter it can hold onto its planets.
Since fluorine has one more proton than oxygen, it has a stronger "pull" on its electrons. It's like having a slightly more enthusiastic tug-of-war rope, and the team with more players (protons) is winning the pull.
The Electron Shell Game
Electrons don’t just float around randomly. They orbit the nucleus in specific energy levels, often called electron shells. Imagine these shells like the different floors of an apartment building. The first floor (closest to the nucleus) is usually the smallest and most tightly packed.
Both oxygen and fluorine have electrons in these shells. Oxygen has 8 electrons, and fluorine has 9. Even though fluorine has an extra electron, it doesn't magically create a whole new, larger floor. Instead, that extra electron is squeezed into the existing shells.

Here’s the key: The electrons in the outermost shell are the ones that really determine an atom’s size. And because fluorine has that extra proton in its nucleus, it’s pulling all its electrons, including the ones on the outer edge, a little bit closer. It’s like if the landlord decided to gently encourage everyone to tidy up and consolidate their belongings, making the whole apartment building feel a bit more compact.
So, while oxygen might have a slightly more relaxed vibe, fluorine’s nucleus is a bit of a taskmaster, keeping those electrons on a tighter leash.
A Tale of Two Elements: Imagine a Tug-of-War
Let’s make this super relatable. Imagine two tug-of-war teams. Team Oxygen has 8 strong players (protons) and 8 opponents (electrons) on the other side. Team Fluorine has 9 strong players (protons) and 9 opponents (electrons) on their side.
Now, the important thing isn’t just the number of players on each side. It’s how tightly those players are gripping the rope. The protons are the ones pulling the rope (electrons) towards them. Since Team Fluorine has one extra player pulling from the center, they can pull the rope (and the electrons) a little bit closer and tighter than Team Oxygen can.

It’s this increased "pull" from the nucleus, due to the extra proton, that shrinks the atom. The electrons are still in pretty much the same "floors" or shells, but they’re being drawn in more intensely, making the whole atom take up less space.
Why Should We Care About Tiny Atoms?
Okay, so fluorine is smaller than oxygen. Big deal, right? Well, it turns out that this little difference in size and electron behavior is super important for how these elements interact with the world around us.
Think about it: if atoms are like LEGO bricks, their size and how they hold onto their outer pieces (electrons) determine how they click together with other bricks. This is the basis of all chemical reactions. The way atoms bond, form molecules, and create everything from water to the complex molecules in our bodies is dictated by these tiny atomic properties.
Fluorine: The Tiny, Fierce Element
Because fluorine is so small and its nucleus has such a strong pull, it's also the most electronegative element. Electronegativity is basically an atom's "greed" for electrons. Fluorine is incredibly good at snatching electrons from other atoms.

This is why fluorine is so useful in things like:
- Toothpaste: Fluoride ions (which are related to fluorine) can integrate into our tooth enamel, making it stronger and more resistant to acid attacks from sugars. It’s like giving your teeth a tiny, protective shield.
- Non-stick Cookware (like Teflon): These amazing pans use compounds that contain fluorine. The strong bonds formed with fluorine make the surface incredibly stable and prevent food from sticking. Imagine a super-slippery surface where nothing can get a good grip – that’s partly thanks to fluorine’s electron-grabbing nature.
- Refrigerants and Propellants: While some of these are being phased out due to environmental concerns, fluorine has played a role in creating substances that can effectively transfer heat or act as aerosol propellants.
Oxygen, being a bit larger and with a less intense nuclear pull, behaves differently. It’s essential for respiration, it’s a key component of water (H2O), and it plays a vital role in combustion. Its properties allow for different kinds of chemical interactions than fluorine.
The Beauty of Small Differences
So, the next time you brush your teeth or cook an omelet without it sticking, you can give a little nod to the fascinating world of atomic physics. That tiny difference in atomic radius between fluorine and oxygen, driven by the simple fact of one extra proton, leads to a cascade of properties that shape our world in profound ways.
It’s a reminder that even the smallest details can have the biggest impact. The universe is full of these subtle variations, and understanding them helps us appreciate the incredible complexity and ingenuity of nature. It’s not just about knowing facts; it’s about marveling at the elegant dance of particles that makes everything possible!